
A Digital Face to Face on Capitol Hill
Rare Disease Legislative Advocacy In A Virtual World
Every year representatives from rare disease organizations from across the country travel to Washington DC. We advocate for legislative issues of importance to the rare disease community by sharing with our legislators and Congressional staff how healthcare policies impact our rare journeys. This annual event also provides a unique opportunity to meet with fellow advocates as a large group. Suppose you can imagine over 800 advocates walking towards our Capitol, descending as a force. The visual is stunning. While February is Rare Disease month, the 2021 Rare Disease On Capitol Hill event was pushed to July in hopes we could return in person.
It’s A Videoconferencing World
Not everyone is comfortable with our new video age. Still, advocates were able to take advantage of a trial run earlier in March. As an organization, the SSRA has embraced the videoconferencing meeting format. Our volunteer group is scattered across the world, so we adapted.
Every advocate meets with their home state Congressional and Senate representatives. Legislators and staff members are especially interested in hearing from their constituents. These are the most impactful meetings. There may not be an advocate available from every district, so often, you may meet with staff members from adjoining Congressional districts. Senate meetings are much larger groups consisting of all of the residents of your state, so often, not everyone can speak.
House Meeting Day- Day 1
Group meetings with Representatives and their staff members are smaller; often, two or three organizations will be scheduled. For example, when I attended RDW 2019, I met with the Legislative Counsel from TX-04 and TX-01. This year, I was assigned four sessions, one with my home district office TX-04, district TX-01, district TX-32, and district TX-05.
I’m not sure if there was confusion with eastern time vs. central, but I found myself the lone advocate twice. Meeting with staffers is always enjoyable and relaxed. They’re engaged and interested in your message. I had a great meeting with Abbie Killian, Legislative Assistant, Congressman Colin Allred (TX-32). Abbie returned my follow-up email immediately, thanking me for my story, the SSRA as an organization, and our support of the STAT ACT (HR 1730).
I chose to focus on a single bill during my meetings instead of covering the lengthy list of healthcare bills being discussed by the larger groups. Abbie promised me their office would begin exploring the possibility of co-sponsoring the bill.
Hello Congressman
The majority of advocacy meetings are always taken with the legislative aide assigned to healthcare issues. Thus, legislative aides are the gatekeepers. However, as I was waiting for Rep. Louie Gohmert’s aide to join, I noticed they were having a bit of trouble connecting. Aides usually are very tech-savvy, so I thought I might be meeting with someone on the mature side. So you can imagine how stunned I was to see Rep. Louie Gohmert connect in person.
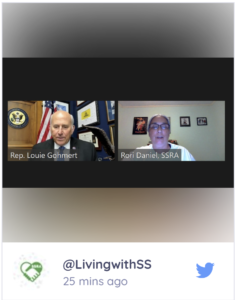
I had notes prepared for a ten-minute talk, but I realized I’d have to fill our entire slot. So our 30-minute meeting turned into a neighborly chat. By beginning with my husband’s personal Superficial Siderosis journey, I shifted into how we became a part of the Superficial Siderosis Research Alliance. This led to a discussion of my recent education in the tangle of clinical trial regulations an organization has to navigate.
And so we arrived at the Speeding Therapy Access Today Act of 2021, H.R. 1730 (S. 670). It was introduced into the House of Representatives and Senate in March 2021. The Stat Act, if passed, will create a division in the FDA dedicated to facilitating rare disease research and expedite the approval of new therapies for the rare disease community. Earlier the 2016 passage of the 21st Century Cures Act allowed for creating a similar division dedicated to oncology treatment and medicines.
Senate Meeting Day-Day 2
Senate meetings are a genuine group effort when you live in a state as large as Texas. In addition, if you’re new to legislative advocacy events, a large group offers you time to become comfortable with the process. The first few minutes are spent introducing the group members and the organization they represent. Finally, the Texas group will have assigned individual volunteers a specific bill to give a short 5-minute description and the importance to the rare disease community.
Why Legislative Advocacy Matters

Sharing our patient and family rare disorder journey while connecting with other organizations helps amplify the message, but anyone can join the conversation. The voices of SSRA friends and supporters are equally important. I’m asking for your help to guarantee our policymakers and legislators are aware of patients’ and caregivers’ challenges. Without hearing from everyone directly, legislators can’t effectively support policies that provide real help.
This upcoming week is a critical opportunity to reinforce the message. When the offices of our congressional leaders receive emails, phone calls, or messages from several citizens on the same topic, they pay attention.
STAT ACT Bullet Points
If you are interested in supporting our message, here is a shortlist of facts.
- The STAT Act has been introduced in both the U.S. House of Representatives and Senate. The legislation has been referred to the House Committee on Energy and Commerce and the Senate Health, Education, Labor, and Pensions Committee.
- The STAT Act authorizes the creation of an FDA Rare Disease Center of Excellence (COE) with distinct programs and authorities to help advance and identify rare disease expertise within FDA. These activities will be carried out using existing funding resources provided to the FDA during the annual appropriations cycle.
- The 21st Century Cures Act gave FDA the authority to develop centers of excellence (described in law as Intercenter Institutes). The STAT Act leverages this existing authority to authorize a center focused on rare diseases while also proposing some specific activities of the Center.
- The Intercenter Institute will implement a program, Accelerating Lifesaving Therapies in Treating Ultra-rare Disease Entities Program, to identify and make recommendations to address current and emerging regulatory science and public policy challenges associated with developing medical products to treat rare diseases or conditions in an individual or very small populations.
Contact Your Legislators
To contact your Members of Congress and ask them to co-sponsor the STAT Act, follow this link to send a message by email or call. You can send a personalized message or send the Everylife Foundations default email.







