
A Rising Global Diagnosis
A Superficial Siderosis Patient Report
A new report on leading patient-centric research for this rising global diagnosis. Review the 2021-2022 global funding landscape and the most current Superficial Siderosis patient demographic statistics.
Introduction
Developing new therapies for a small population rare disorder is challenging. Funding concerns force hard decisions. Discover innovative interventions meant to address the worst symptoms, bringing a potential improvement to the quality of life, search for a cure? Researchers are faced with extensive regulatory requirements and the need to address viewpoints from healthcare payers, care providers, and patients regarding the efficacy and long-term safety of all new therapies. For this reason, the traditional clinical trial model needs to be redesigned to meet the needs of a small global population. Patient registries must modernize the ability to collect data, develop real-world evidence, distribute and share the information, and keep pace with development and regulatory needs. Patient-led research advisory groups can amplify the patients’ voices and shape the research agenda towards the needs of those who matter. The Superficial Siderosis Patient Registry is a global patient and caregiver-led effort designed to connect our community members from every corner of the world.
Background
Background
Infratentorial superficial siderosis, type 1 (classical) (iSS)(SS), commonly identified as Superficial Siderosis of the Central Nervous System is an extremely disabling progressive disorder affecting the brain and spinal cord. Continuing or recurrent long-term bleeding into the subarachnoid space results in an accumulation of hemosiderin on the surface of the brain and pia mater from circulating cerebrospinal fluid. SS patients will present with one or more symptoms- sensorineural hearing loss, ataxia, and myelopathy with pyramidal signs. Recent documentation of cognition and mood issues has identified these areas as an additional cause for concern. ¹ Classified as an ultra-rare disorder by the National Organization of Rare Disorders (NORD) the global incidence of confirmed diagnosed cases was thought to be one in three million. Patient registry enrollment places the occurrence rate at one person per million in the United States. While technological improvements in magnetic resonance imaging (MRI) have increased the confirmed diagnosis rate, the actual incidence of SS is suspected to affect a more significant population than is currently identified.
Source
Superficial siderosis is classified as an acquired disorder. Is there a genetic link? A connection has yet to be explored. Identifiable bleed sources are abnormalities resulting from recurring brain bleeds (chronic suboccipital hematomas), sacs protruding from the spinal column (meningoceles), or spinal surgery tears resulting in
pseudomeningoceles. Other causes include root avulsions, epidural cyst removal, intradural cranial surgery, brain tumors, vascular abnormalities, fragile capillary regrowth after brain surgery, nerve damage (brachial plexus injury), head injury, or bone marrow exposed to the spinal (intrathecal) space. Hemolysis ruptures blood cells in the cerebrospinal fluid creating a heme overload that triggers Bergman glia and microglial cells into producing the enzyme heme oxygenase 1. HO-1 breaks down heme molecules resulting in the release of free-iron molecules, carbon dioxide, and biliverdin. Astrocytes release ferritin proteins to contain the
free-iron molecules; the resulting product is hemosiderin. The insoluble nature of hemosiderin allows gravity to draw it to the subpial surfaces in the infratentorium and spinal column, attaching to the pia mater with substantial accumulations on the cerebellum. Cranial nerve sections, brainstem, and the spinal cord may also become encased in hemosiderin. Long-term exposure to free-iron molecules released from the hemosiderin layer is toxic to the underlying neural tissue.
¹ in a recently published communication, Chan et al. (2021) found “cognitive impairment was present in 8/16 (50%) of patients: executive dysfunction was the most prevalent (44%), followed by impairment of visual recognition memory (27%)”. The conclusion was that neuropsychological disturbances and mood disorders are more widespread in is than previously documented.
Therapeutic Options
Superficial siderosis differs from more complex neurological diseases and disorders such as Multiple Sclerosis, Parkinson’s, ALS, or multiple system atrophy because the neuronal injury originates from a singular source. While co-morbidities may influence individual cases, the primary source of disability would be reduced with
the discovery of a successful therapeutic intervention. Standard therapy continues to involve the repair of an identified active bleed site to prevent persistent blood infiltration. ² Iron chelation has been attempted with moderate success but not all patients are able to physically tolerate the intervention. For patients currently undergoing chelation therapy, neuronal injury progression continues during an elimination process that may take years if successful. Suppression of hemosiderin deposition off the subpial surface is essential to potentially interrupt cerebellar atrophy and neural damage. There are currently no effective alternate therapies to combat or slow neural damage progression either during iron chelation therapy or during the natural course of the disorder.
² Kondziella et al. (2015) concluded, “Surgical therapy in superficial CNS siderosis is rarely achieved. We suggest that prospective, large-scale multicentre studies are needed to search for non-surgical therapies that reverse (or prevent) ongoing neurotoxicity due to accumulating iron toxicity.”
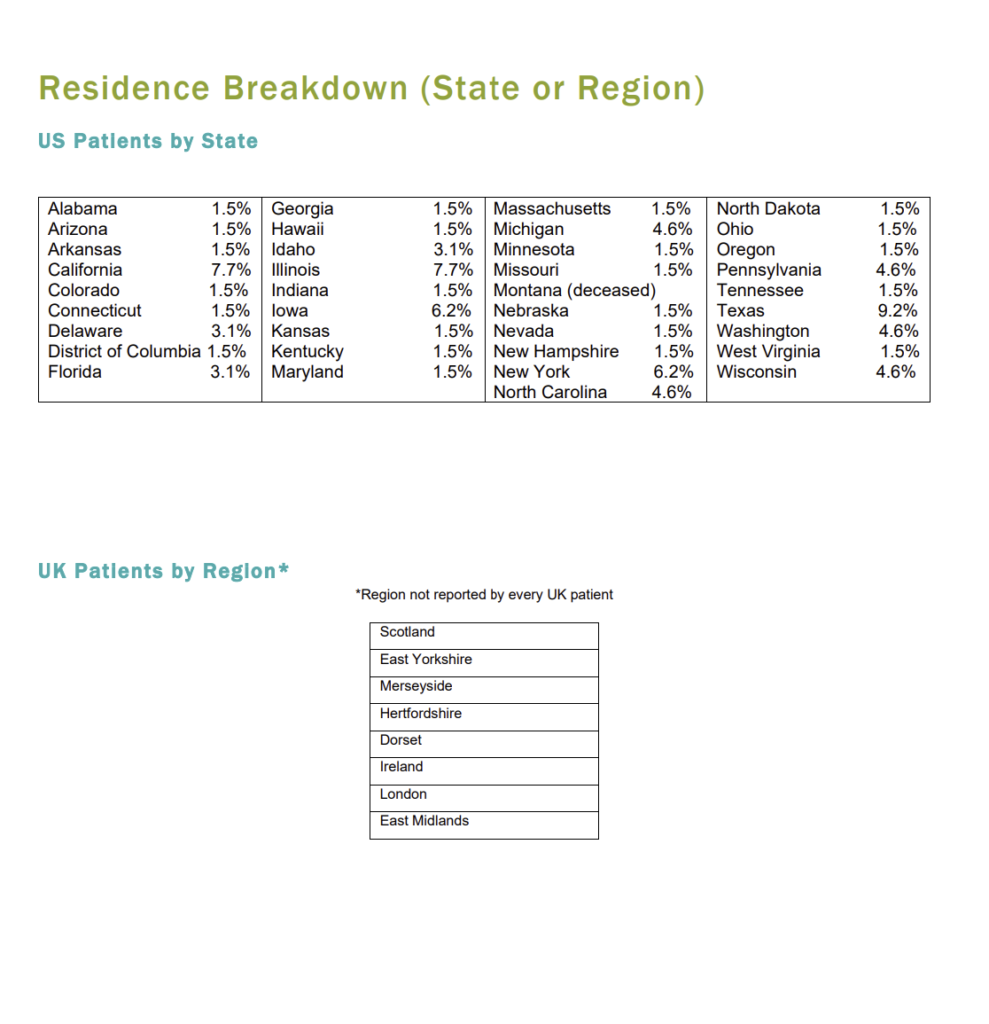
Patient Registry Statistics


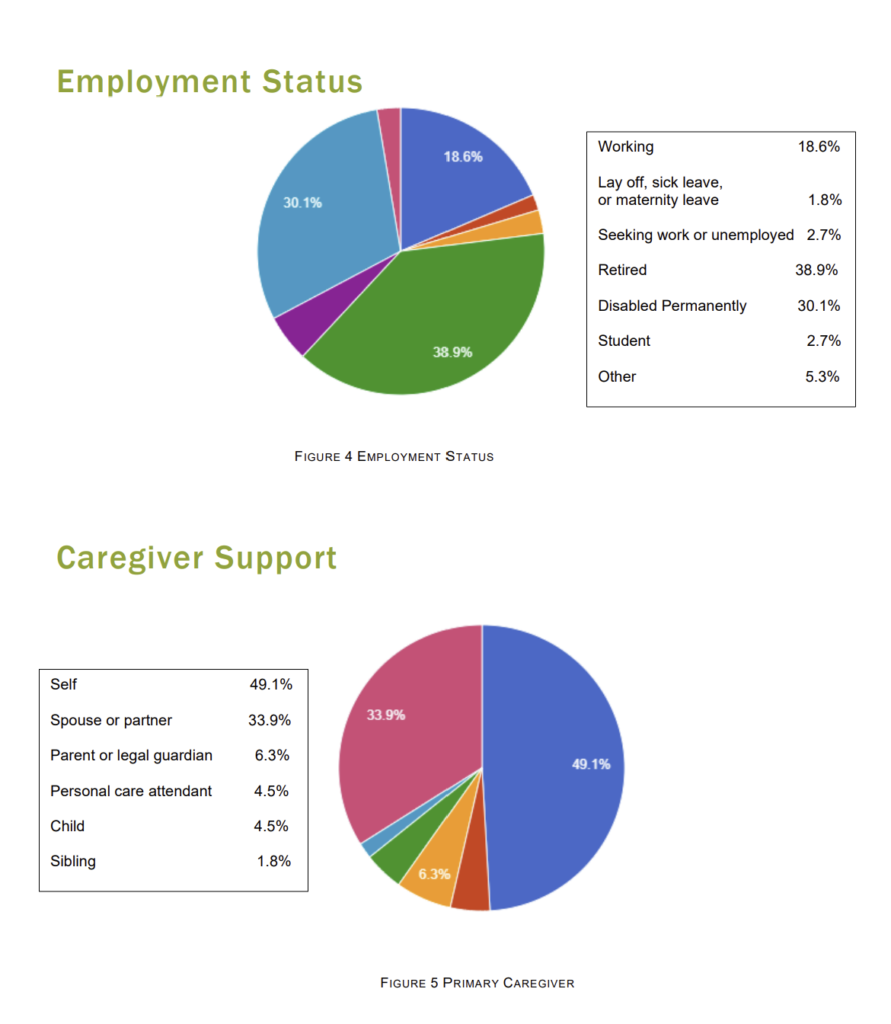



Patient Opinion Poll
Question: Choose the top three research barriers
• Lack of Interest in Small Population Rare Disease/Disorders 88.9%
• Little or No Government Support 62.0%
• Small Public (Government) Research Funding Allotments 20.4%
• Lack of Private Investment Funding 22.2%
• Low Number of Interested Researchers 32.4%
• Low Physician/Researcher Understanding 45.4%
• Lack of Patient Involvement 2.8%
• Low Interest in the Patient Point of View 2.8%
Global Research Outlook
UK Funding Reductions
In the past, the allotment of Official Development Assistance (ODA) funds to UK Research and Innovation (UKRI) has allowed the UK a significant role in tackling global rare disease and disorder challenges. The 2020 budget across UKRI for ODA research projects was £422M. The 2021 budget has been reduced to £125M, necessitating significant budgetary cuts. This extraordinary action is causing the curtailing or even termination of existing projects. The damage this reduction will bring to current operational projects will put many at severe risk. In addition, joint projects with global partners who rely on the support of the UK research collaborators put long-time partnerships in jeopardy. Superficial siderosis research will feel the effects of this reduction most strongly as small population rare
disorders will no longer be afforded priority when future allocations are made.
US Fiscal Year 2022 Proposed Funding
The US released the FY2022 budget proposal, which included a recommendation for an increase in the budget for the National Institutes of Health (NIH) by $2.5 billion and providing $6.5 billion for the establishment of a new science and technology initiate called ARPA-H, a $3.6 million increase for the Food and Drug Administration (FDA), with $500 million exclusively for funding the FDA under the 21st Century Cures Act. This budget proposal recommendation must pass congressional approval before implementation.
CA 2021-22 Health Plan
The Canadian Health Minister announced that Canada was developing a national strategy on high-cost drugs for
rare diseases and establishing a transition office to oversee the creation of a Canadian Drug Agency.
Patient Registry
Areas Needing Improvement
Visibility
A search of published reports reveals documented cases of SS have been identified in almost every geographical area of the world. High instances have been documented in Italy, France, Japan, South Korea, Brazil, Syria, and Iran. Increased outreach into the medical community in these countries is necessarily better to understand the global population of diagnosed superficial siderosis patients.
Natural History Study
Implementation of a long-term superficial siderosis natural history study in coordination with the SS Brain Tissue donation program is a high priority. The patient registry has the infrastructure to safely house data collection and offers remote access to all researchers.
Conclusion
Until now, the UK has led in the number of active superficial siderosis research projects. Continued government funding for existing rare disease research projects in the UK is now in a precarious state. Superficial siderosis may have a minimal footprint, but the loss of support will slow or stop the promise of progress.
This patient registry hopes are to redefine the traditional research model into a viable remote electronic network capable of connecting with dual global communities- research and patient. While we believe patient-centric exploration will lead to an improved future, we also recognize that many research questions need to be investigated.
Is there a genetic component? Why is the majority of the world population not affected by this devastating disorder? What makes such a small population at risk? How can the sole therapeutic option be improved, made affordable, made safer? How do we identify new therapies? We will only achieve these goals by expanding our research network into the virtual world, participating in multi-country collaborative projects, and developing solutions.
Data Source
The Superficial Siderosis Patient Registry provided the data used to complete this demographics report from 150 volunteer respondents who completed a survey intended for advocacy and outreach projects.
Acknowledgements
- Everylife Foundation
- Superficial Siderosis Research Alliance
- The Silent Bleed Superficial Siderosis Charity (UK)
- Superficial Siderosis Patient Registry
The SSRA would like to acknowledge and thank the SS patient community for their help. For sharing your voice.
Download


