
Biobanks Are Transforming Research
The Importance of Biobanks in Rare Disorders
The design of the Superficial Siderosis Patient Registry intentionally includes the capability to reach every corner of the world. With our upgraded capacity to record patient submitted information, the time has come to increase our global enrollment outreach program. To begin the next step in our research evolution. Biomarkers are an essential part of natural history studies for rare disorders. Because biomarker development is such a high priority, we must begin the development of a biobank.
Natural History Study
Designing a Superficial Siderosis Natural History study has always been a top priority; to go beyond the simple recording and cataloging of symptoms and basic patent demographics. Natural history studies are critical to support the development of safe and effective drugs and biological products for any rare condition.
Natural history is customarily defined as the progression a disease or disorder takes. Without any intervention from the onset of symptoms and during the advancement of the condition. A preplanned observational study intended to track the course of the natural course of a disease. It also helps to track genetic, environmental, and patient demographics. Additional details will be followed, like the current care plans or new out-of-the-ordinary care that alters the progression and treatment medications related to the disorder. Patient registries have long been the standard platform to acquire the data for natural history studies.
Why Biobanks Are Important
A 2018 review of rare disease patient registries compared 14 registries not associated with biobanks, nine registries associated with a biobank infrastructure, and six additional biobank resources. A patient registry and biobank complement each other by providing the entire picture to researchers. A patient registry can help create a clear understanding of the natural history of a condition. This understanding will help lead to developing best practices and improving patient outcomes. However, when the data from a patient registry is used in combination with biobank samples, breakthroughs can happen to lead to clear strategies and treatments.
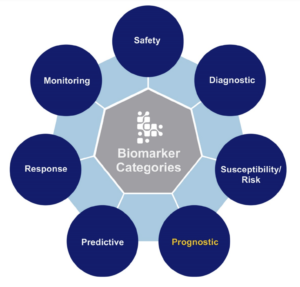
Diagnostic biomarkers in other rare conditions help arrive at a quicker diagnosis and correct disease management, preventing unnecessary treatments due to misidentification. For example, identifying superficial siderosis is pretty straightforward; the MRI proof is visible right before your eyes. Unfortunately, some physicians still remain unconvinced superficial siderosis is the source of their patient’s symptoms and progression. This desire to rule out every possibility sometimes leads to an expensive and long string of tests.
When the Superficial Siderosis Patient Registry connects with a biobank infrastructure, researchers will be allowed to identify and validate biomarkers. In addition, by interpreting the complete genetic and molecular profiles of superficial siderosis patients, we will gain the ability to create new therapeutic strategies. Therefore, biobanks should be a priority for all rare conditions.
What Are Biomarkers
Biomarkers are scientifically measurable samples entirely different from self-reports on how a person feels or functions. Instead, these collected samples measure the presence or progression of a condition or clinical trial treatment effects. Biomarkers may be molecular, histologic, radiographic, or physiological. For example, biomarkers may range from blood pressure readings, heart rate, basic metabolic panels, and MRI scans to intricate histological and genetic testing of blood or other tissues.
- Molecular have physical properties to allow a measurement of biological samples
- Radiographic are obtained from imaging studies
- Histologic show biochemical or molecular change in cells, tissues or fluids
- Physiologic are measurment and recording of of body processes

Creating A Repository
A biorepository can store a broad array of collected biological samples. Our partnership with Dr. Michael Levy, the Superficial Siderosis Clinic and Research Laboratory, and Massachusetts General Hospital will provide the resources needed to establish a repository for biological samples collected from participating superficial siderosis patients.
A Global Effort
We recognize the limitations facing small patient groups, and for this reason, our project will be a multi-country effort. This project will require participation from patients and their families and a coordinated effort between outside institutions and countries to reach our goals.
What does this mean in practical terms? First, the lack of coordinated global regulations regarding biomarker and genetic sampling in natural history studies means we will need to carefully assess the regulatory environment of every prospective participant’s home country during the planning phase.
A biomarker once qualified for a particular context of use, should be publicly available and can be applied in any drug development program for the qualified context of use
FDA.gov
For example, in France, approval from the Ethics Committee (EC) and Regulatory Authority (RA) is needed for studies involving biomarker genetic sample collection. In other countries, where regulatory requirements for genetic testing are less structured, a single regulatory committee may oversee approval. We want to include as many countries as possible, so every superficial siderosis patient is allowed an opportunity to be involved.
Identifying Research Goals
Our most important purpose is to go beyond the researchers’ need to understand the how and why of superficial siderosis. To develop safe and effective treatments for not only slowing down the progression but eventually stopping the progression. Just imagine the possibilities of a genetic link. One day there might even be a test developed to screen and identify those at risk of developing superficial siderosis before it happens.
You may not have noticed the SSRA website has a newly added RESEARCH section. You’ll find the latest superficial siderosis related publications and studies from around the world posted, updates throughout the year on our progress, research-related blog posts, and a link to the patient registry.
Preparing The Way
The study design is now underway. We have a two-part plan for funding. First, the SSRA will continue fundraising activities to
- Support and increase the patient registry data storage capacity- data storage is billed on a sliding upward scale ($4,300 annually at current level)
- Increase enrollment through a global recruitment campaign (unknown)
- Deliver the third installment to Massachusetts General Hospital in early 2022 ($25,000)
- Continued fundraising for the purchase of 3-year supply of antioxidants ($45,000)
- Professional grant application assistance ($2,000 x 2 if nessecary)
Secondly, the funding needs of a multi-country study are substantial. Our plan includes
- Application to the FDA Rare Disease program for a Natural History Grant
- Application to the FDA Rare Disease for New Therapeutics to conduct the Antioxidant Study through Massachusetts General Hospital
Additional plans include sponsoring a multi-country round table video conference bringing researchers, neurologists, and associated symptom specialties together to discuss and plan a global standard of care for superficial siderosis.
New diagnostic and therapeutic strategies are urgently needed to manage superficial siderosis. Nevertheless, the day will come when new treatments can prevent neural tissue damage. All of us will have played a significant role.
2 thoughts on “Biobanks Are Transforming Research”
Leave a Reply
You must be logged in to post a comment.




It seems to be a great start that has little answer to the global aspects of a global community. The US feedback is good but rat.her short on the feedback system then other countries do not have a linkage apart from self reporting and has little connection to the issue of the article. Sorry Rori
Our relationship with Mass General Hospital will simplify the coordination between countries. Many of their studies have multi-country participants, so there is an approval process already in place. I know it must seem like there will never be progress for those who live in Australia, but that’s why we are working to solidify connections with the medical field there. For example, the Head of Neurology in Victoria is very keen to become involved in our efforts.
Until recently, there were only three known SS patients in Australia, you being one of them, but we now have connected with a total of nineteen. Our outreach efforts are paying off.
We are waiting for board approval of our application to join EURORDIS. This will smooth the way for our registry to be listed in the World Rare Disease and Disorder Directory and on ORPHANET.